October 2023
La Vague n°79
Special A3P Congress 2023 : Annex 1, Environmental and process monitoring.
Table of Contents
- EU GMP Annex 1 (2022): Aseptic Process Simulation (APS). New challenges for the pharmaceutical industry?
- How to evaluate pyrogenic risk in an injectable pharmaceutical process? Decision-making tool
- Introduction to the dual chamber systems (DCS) filling process
- Implementation of decontamination processes in the pharmaceutical industryentation of decontamination processes in the pharmaceutical industry
- Container Closure Requirements in the New EU GMP Annex 1 - Enabling Compliance with a Holistic Science-Based Approach
- Improving the efficiency & reliability of AVI qualification: Determining the optimal number of runs with the KNAPP method
- Case Study: Effective Sterile Powder Transfer for Parenteral Drug Products
How to evaluate pyrogenic risk in an injectable pharmaceutical process? Decision-making tool
In 2016, Chapter 5.1.10 of the European Pharmacopoeia “Guidelines for using the test for bacterial endotoxins” changed, with notably the addition of the requirement to carefully evaluate the risk of the presence of non-endotoxin pyrogens (NEPs) in the substance or the product to be tested in order to be able to use the bacterial endotoxin test as the sole test for pyrogens.

During the A3P 2017 conference with the ocean as a backdrop, this chapter was the topic of discussion in our group of microbiologist friends from different companies. During the course of the discussion, numerous questions emerged, such as: “What are the different pyrogenic substances? How can they be detected? What are the advantages and disadvantages of the different detection methods? How to evaluate the risk of the presence of pyrogens for a new product?
This article presents an example of an approach that can be used in the case of injectable products and made available to manufacturers, while complying with regulatory recommendations.
1. The large family of pyrogenic substances
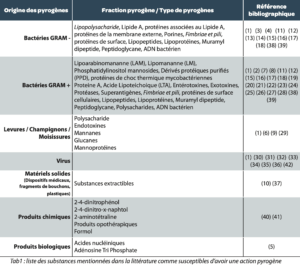
An exhaustive bibliographic search allowed us to draw up a list of the following substances mentioned in the literature as liable to have a pyrogenic action (See Table 1).
It is notable that very many substances, of varying natures although mainly derived from living beings, are recognized as having a pyrogenic effect. Within these substances, endotoxins derived from Gram-negative bacteria are known and recognized as those with the highest pyrogenic activity. This probably explains why pyrogens and bacterial endotoxins are sometimes confused.
2. Detection of pyrogens: the advantages and disadvantages of different methods
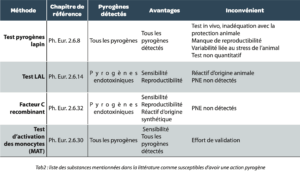
Different methods are described in the European Pharmacopeia for the detection of pyrogens. Table 2 presents the advantages and disadvantages of these techniques. In recent years regulations have encouraged the gradual elimination of pyrogen tests on rabbits, in order to dispense with animal testing.
Moreover the use of recombinant reagents is also introduced as an alternative to reagents of animal origin.
3. Method for evaluating the risk of the presence of pyrogens for a new product
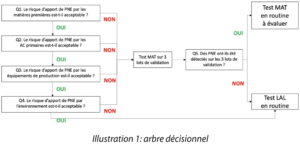
Our reflection and our lively and constructive exchanges during the face-to-face working sessions, at a time when this was the norm (2018), led us to define the questions to ask and the information to compile, to standardize and justify the choice of the type of test to be applied. The fruit of our work is summarized in the decision tree (illustration 1).
With the aim of having an approach at once structured and reproducible we redefined the response criteria to each of the essential questions. Quantitative criteria are not indicated in this article, as it is each manufacturer’s responsibility to define them on the basis of the risks inherent in the process evaluated.
With the aim of having an approach at once structured and reproducible we have redefined the response criteria to each of the essential questions. Quantitative criteria are not indicated in this article, as it is each manufacturer’s responsibility to define them on the basis of the risks inherent in the process evaluated.
Question 1. Is the risk of the introduction of NEPs by raw materials acceptable ?
As one of the major sources of the introduction of NEPs in materials is linked to the presence of microorganisms other than Gram-negative bacilli, particular attention is paid to controlling the bioburden of materials used in the manufacturing process.
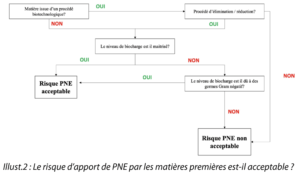 |
| Illustr. 2: Is the risk of the introduction of NEPs by raw materials acceptable? |
Table 2: list of substances mentioned in the literature as liable to have a pyrogenic action
Question 2. Is the risk of the introduction of NEPs by primary packaging items acceptable ?
This question may take into account the existence or absence of a validated process for reducing the pyrogen burden at the supplier’s premises during the manufacturing process (10:37). Note that washing is a bioburden reduction process that can also reduce the pyrogen burden (specific validation to be conducted).
Question 3. Is the risk of the introduction of NEPs by production equipment acceptable ?
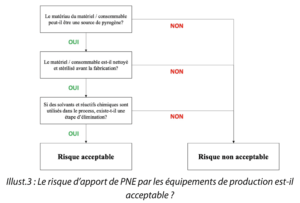 |  |
Illustr. 3: Is the risk of the introduction of NEPs by production equipment acceptable?
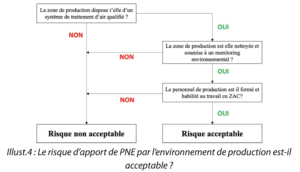
Question 4. Is the risk of the introduction of NEPs by the production environment acceptable ?
Illustr. 4: Is the risk of the introduction of NEPs by the production environment acceptable?
Question 5. Detection of pyrogens by the MAT test on 3 validation batches ?
In the event of a negative response to questions Q1 to Q4, the MAT test may be performed on 3 validation batches. If pyrogens are detected, an investigation must be conducted to define the origin of the presence of the pyrogen(s). Depending on the results of the investigation, the routine implementation of a mat test may be envisaged.
4. Conclusion
The tool presented is an example of an approach that can be used to respond to the problem of the possible presence of pyrogens in injectable products. This reflection should be included when developing a new product. The recent appearance of Annex 1 has only accentuated the need to conduct risk analyses at all levels in order to reduce the risks of microbiological, particulate and a pyrogenic contamination. This will be also reinforced with the upcoming appearance of a new chapter of the European Pharmacopoeia, Chapter 5.1.13 Pyrogenicity, currently under review for comments.
This working group, set up spontaneously, is further proof of the extent to which all manufacturers are confronted by similar problems, and that working together enriches us all.
Share the article






References
1 T. SANDLE (2015), Assessing Non-endotoxin Microbial Pyrogens in relation in pharmaceutical processing, Institute of Validation Network, March 2015.
2 K. W. BRUNSON AND DENNIS W. WATSON (1974), Pyrogenic Specificity of Streptococcal Exotoxins, Staphylococcal Enterotoxin, and Gram-Negative Endotoxin, Department of Microbiology, University of Minnesota Medical School, Minneapolis, Minnesota 55455, April 1974.
3 T. SANDLE (2012), Pyrogens, endotoxin and the LAL test: An introduction in relation to pharmaceutical processing, Global BioPharmaceutical Resources, Newsletter, May 2012.
4 P. GRANDICS (2000), Pyrogens in parenteral pharmaceuticals, Pharmaceutical Technology, April 2000 24(4):26-34.
5 R. CHARONNAT, P. LECHAT (1950), Recherches sur la nature des pyrogènes des solutés injectables, Mémoires présentés à l’Académie de Pharmacie-séance de mai 1950, Annales Pharmaceutiques Françaises, 1950; 8(3):171-81.
6 A. I. BRAUDE, J. MCCONNELL, and H. DOUGLAS (1960), Fever from pathogenic fungi. Journal of Clinical investigation, 1960, Aug; 39(8): 1266–1276.
7 I. GIMENES, C. CALDEIRA, O. A. FRANCA PRESGRAVE, W. CORREA DE MOURA, M. H. SIMOES VILLAS BOAS (2015) Assessment of pyrogenic response of lipoteichoic acid by monocyte activation test and rabbit pyrogen test, Regulatory Toxicology and Pharmacology. 73 (2015) 356-360.
8 A. R. HAUSER, D. l. STEVENS, E. l. KAPLAN and P. M. SCHLIEVERT (1991), Molecular Analysis of Pyrogenic Exotoxins from Streptococcus pyogenes Isolates Associated with Toxic Shock-Like Syndrome, Journal of Clinical Microbiology, Aug. 1991, p. 1562-1567 Vol. 29, No. 8.
9 S. NEYTCHEFF (1963), The pyrogenic effect of polysaccharides of C. albicans, C. tropicalis, C. pseudotropicalis and C. krusei , Zentralblatt für Bakteriologie. 1963 Sep; 190:132-8.
10 P. MICHON, A. LARCAN, J.-M. PICARD, C. KLING (1960), Les accidents perfusionnels de type pyrogénique: Contribution à l’étude de la toxicité éventuelle de substances plastiques utilisées dans les dispositifs de perfusion, Transfusion, 1960, Volume 3, Issue 2, Pages 131-144.
11 C.A. DINARELLO, R.J. ELIN, L. CHEDID, and S. M. Wolff (1978), The pyrogenicity of the synthetic adjuvant muramyl dipeptide and two structural analogues. Journal of Infectious Diseases, 1978, (198) 138, 760–767.
12 L. JOHANNSEN, J. WECKE, F. OBAL Jr and J.M. KRUEGER (1991), Macrophages produce somnogenic and pyrogenic muramyl peptides during digestion of staphylococci. American Journal of Physiology, 1991, 260, R126–R133.
13 D. FUMAROLA, I. MUNNO, C. MARCUCCIO and G. MIRAGLIOTTA (1986), Endotoxin-like activity associated with Lyme disease Borrelia, Zentralblatt für Bakteriologie, Mikrobiologie und Hygiene, 1986, 263, 142–145.
14 A. THOMSEN, H. LOPPNOW (1995). Cytokine production by mononuclear cells following stimulation with a peptide-containing, endotoxin-free Escherichia coli extract. Arzneimittelforschung, 1995 May;45(5):657-61.
15 R. J. ELIN and A. E. UTTER (1980), Positive Limulus amoebocyte lysate reactions with polyriboinosinic acid × polyribocytidylic acid. Journal of Clinical Microbiology, 1980, 12 (4), 502–505.
16 S.J. WONN and M.T. LIN (1993), Endogenous pyrogen formation by human blood monocytes stimulated by polyriboinosinic acid × polyribocytidylic acid. Experientia, 1993, Feb 15;49(2):157-9.
17 J.S. COWDERY, J.H. CHACE, A.K. YI, A.M. KRIEG (1996), Bacterial DNA induces NK cells to produce IFN-gamma in vivo and increases the toxicity of lipopolysaccharides. Journal of Immunology, 1996, Jun 15;156(12):4570-5.
18 T. SPARWASSER, T. MIETHKE, G. LIPFORD, K. BORSCHERT, H. HÄCKER, K. HEEG, H. WAGNER (1997), Bacterial DNA causes septic shock, Nature, 1997 Mar 27;386(6623):336-337.
19 H. WEXLER and J.D. OPPENHEIM (1979), Isolation, characterization, and biological properties of an endotoxin-like material from the Gram-positive organism Listeria monocytogenes. Infection and Immunity, 1979, 23, 845–857.
20 J.G. VALLEJO, C.J. BAKER, M.S. EDWARDS (1996), Roles of the bacterial cell wall and capsule in induction of tumor necrosis factor alpha by type III group B streptococci. Infection and Immunity, 1996, 64(12):5042-5046.
21 O. TOIEN and J.B. MERCER (1996), Thermosensitivity is reduced during fever induced by Staphylococcus aureus cell walls in rabbits. Pflugers Archiv, European Journal of Physiology, 1996, 432, 66–74.
22 S.P. HACKETT, D.L. STEVENS (1992), Streptococcal toxic shock syndrome: synthesis of tumor necrosis factor and interleukin-1 by monocytes stimulated with pyrogenic exotoxin A and streptolysin O. The Journal of Infectious Diseases 1992 May;165(5):879-885.
23 D. FITZGERALD, I. PASTAN (1993), Pseudomonas exotoxin and recombinant immunotoxins derived from it, Annals of the New York Academy of Sciences, 1993, Jun 23;685:740-745.
24 S. BHAKDI, F. GRIMMINGER, N. SUTTORP, D. WALMRATH, W. SEEGER (1994), Proteinaceous bacterial toxins and pathogenesis of sepsis syndrome and septic shock: the unknown connection. Medical Microbiology and Immunology, Berlin, 1994, 183, 119–144 (published erratum appears in Medical Microbiology and Immunology, Berlin 183, 343–344).
25 S. HOULDSWORTH, P.W. ANDREW AND T.J. MITCHELL (1994), Pneumolysin stimulates production of tumor necrosis factor alpha and interleukin-1 beta by human mononuclear phagocytes. Infection and Immunity, 1994, 62, 1501–1503.
26 T. MURAI 1, Y. NAKAGAWA, Y. OGAWA (1996), Potentiation of lethal endotoxin shock by streptococcal pyrogenic exotoxin in rabbits: possible relevance of hyperreactivity of macrophages to endotoxin. FEMS Immunology and Medical Microbiology, 1996, 13(4), 269–272.
27 L.A. CARSON, L.A. BLAND, L.B. CUSICK, M.S. FAVERO, G.A. BOLAN, A.L. REINGOLD and R.C. GOOD (1988), Prevalence of nontuberculous mycobacteria in water supplies of hemodialysis centers. Applied Environmental Microbiology 1988, 54(12), 3122–3125.
28 G. RAWADI, S. ROMAN-ROMAN (1996), Mycoplasma membrane lipoproteins induced proinflammatory cytokines by a mechanism distinct from that of lipopolysaccharide. Infection and Immunity, 1996, 64(2):637-643.
29 V.S. Barwick, M.H. Wooten, J.F. Bradfield, R.D. Myers (1994), Fever of unknown origin: due to C. albicans or other fungi acting on the hypothalamus? Brain Research, 1994 Jan 28;635(1-2):1-8.
30 D.W. BARRY, R.E. MAYNER, H. D. HOCHSTEIN, R.C. DUNLAP, S.C. RASTOGI, J.E. HANNAH, R.J. BLACKBURN, J.L. SULLIVAN, R.J. GERETY (1976), Comparative trial of influenza vaccines. II. Adverse reactions in children and adults. American Journal of Epidemiology 1976, 104 (1), 47–59.
31 K.J. JAKEMAN, C.R. BIRD, R. THORPE, H. SMITH, C. SWEET (1991), Nature of the endogenous pyrogen (EP) induced by influenza viruses: lack of correlation between EP levels and content of the known pyrogenic cytokines, interleukin 1, interleukin 6 and tumour necrosis factor. Journal of General Virology, 1991, 72, 705–709.
32 S. BECKER, J. QUAY, J. SOUKUP (1991), Cytokine (tumor necrosis factor, IL-6, and IL-8) production by respiratory syncytial virus-infected human alveolar macrophages. Journal of Immunology 1991, 147(12):4307-4312.
33 A.M. ALLUWAIMI, H. SMITH, C. SWEET (1994), Role of surface glycoproteins in influenza virus pyrogenicity. Journal of General Virology 1994, 75, 2835–2840.
34 D.M. CHANG, M.F. SHAIO (1994), Production of interleukin-1 (IL-1) and IL-1 inhibitor by human monocytes exposed to dengue virus Journal of Infectious Diseases.1994, 170 (4), 811–817
35 M. KUROKAWA, M. IMAKITA, C.A. KUMEDA, K. SHIRAKI (1996), Cascade of fever production in mice infected with influenza virus. Journal of Medical Virology 1996, 50 (2), 152–158.
36 G.E. PRICE, R.J. FENTON, H. SMITH, C. SWEET (1997), Are known pyrogenic cytokines responsible for fever in influenza? Journal of Medical Virology 1997, 52 (3), 336–340.
37 K.M. MILLER, J.M. ANDERSON (1988), Human monocyte/macrophage activation and interleukin 1 generation by biomedical polymers. Journal of Biomedical Material Research 1988, 22 (8), 713–731.
38 B. HENDERSON, M. WILSON (1996), Cytokine induction by bacteria: beyond lipopolysaccharide, Cytokine 1996, 8 (1)269-282.
39 M. LAUDE-SHARP, N. HAEFFNER-CAVAILLON, M. CAROFF, F. LANTREIBECQ, C. PUSINERI, M.D. KAZATCHKINE (1990), Dissociation between the interleukin 1-inducing capacity and Limulus reactivity of lipopolysaccharides from gram-negative bacteria. Cytokine. 1990, 2(4):253-258.
40 R. CHARONNAT, P. LECHAT (1951), Research on the nature of pyrogens in injection solutions. II. Attempts of quantitative determination of pyrogenic substances; pyrogenic index Annales Pharmaceutiques Françaises. 1951, 9(1):22-30.
41 L. Dubreuil et Ch. Romond (1981), Le pyrogène bactérien. Bulletin de la société de pharmacie de Lille 1981, 1:11-18
42 J.H. GONG, H. SPRENGER, F. HINDER, A. BENDER, A. SCHMIDT, S. HORCH, M. NAIN, D. GEMSA (1991), Influenza A virus infection of macrophages: enhanced tumor necrosis factor alpha (TNF-alpha) gene expression and lipopolysaccharide- triggered TNF-alpha release. Journal of Immunology, 1991, 147 (10), 3507–3513.