October 2023
La Vague n°79
Special A3P Congress 2023 : Annex 1, Environmental and process monitoring
Sommaire
- EU GMP Annex 1 (2022): Aseptic Process Simulation (APS). New challenges for the pharmaceutical industry?
- How to evaluate pyrogenic risk in an injectable pharmaceutical process? Decision-making tool
- Introduction to the dual chamber systems (DCS) filling process
- Implementation of decontamination processes in the pharmaceutical industryentation of decontamination processes in the pharmaceutical industry
- Container Closure Requirements in the New EU GMP Annex 1 - Enabling Compliance with a Holistic Science-Based Approach
- Improving the efficiency & reliability of AVI qualification: Determining the optimal number of runs with the KNAPP method
- Case Study: Effective Sterile Powder Transfer for Parenteral Drug Products
Implementation of decontamination processes in the pharmaceutical industry
This article is based on feedback from members of the A3P cleaning CIG and aims to guide readers in implementing processes for the decontamination of premises and equipment in connection with the regulatory texts associated with this topic. This feedback reflects good practices that have been successfully implemented in the pharmaceutical industries for sterile and non-sterile production.
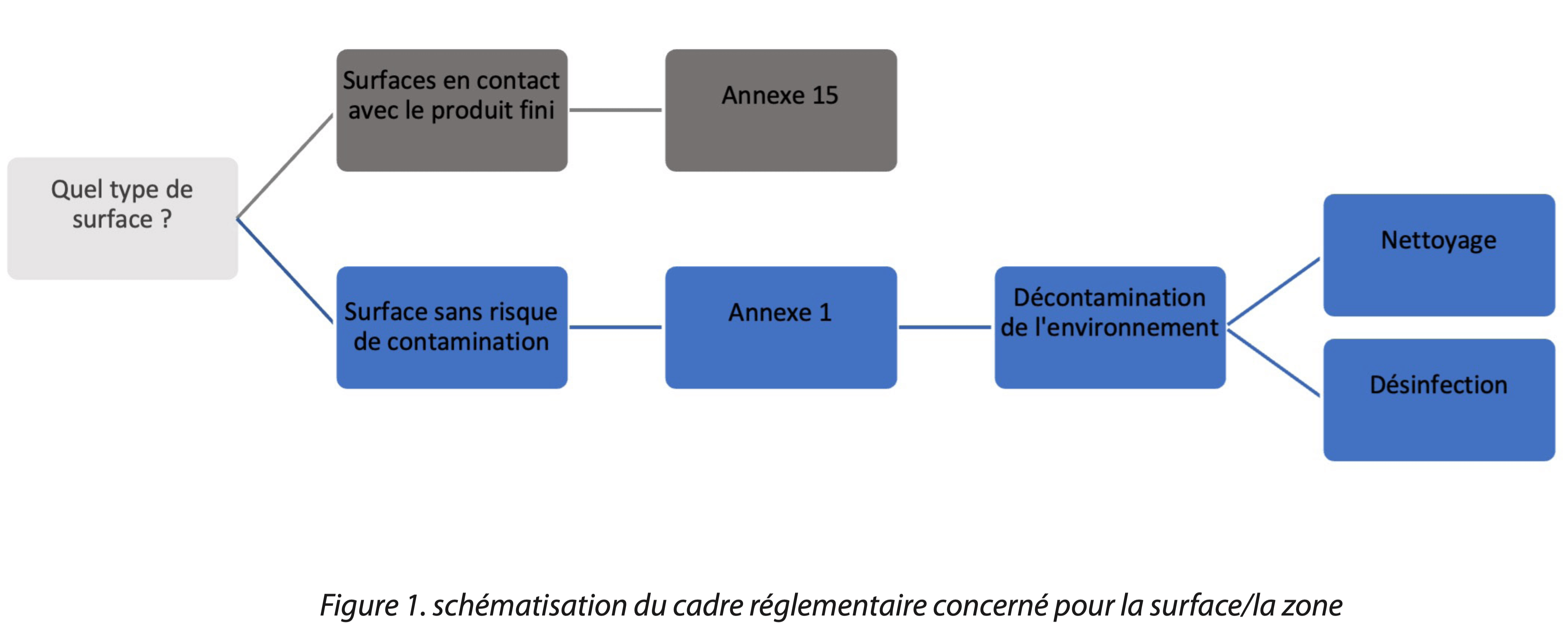
The source text of this article is mostly derived from the guidelines “The Rules Governing Medicinal Products in the European Union – Volume 4 EU Guidelines for Good Manufacturing Practice for Medicinal Products for Human and Veterinary Use”. The recommendations of this text have been adapted so that each manufacturer (classified/unclassified area) can find their bearings easily. This article concerns surfaces with no contamination risk for the finished product (blue tree diagram in Figure 1).
As a reminder, the definitions of terms in this article are as follows, based on the following guidelines: “The Rules Governing Medicinal Products in the European Union Volume 4 EU Guidelines for Good Manufacturing Practice for Medicinal Products for Human and Veterinary Use“.
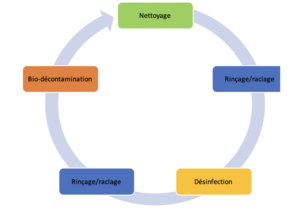
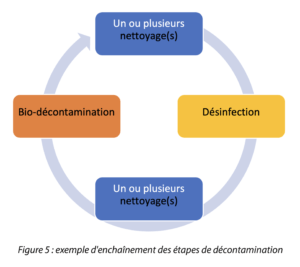
The complete cycle may be illustrated as in this illustration.
This standard decontamination sequence may be applicable to all types of surfaces; the methods to be applied differ according to the surface types that are generally categorized in the following manner:
- spaces and large surfaces
- equipment / small, fixed surfaces (cleaned in the area) mobile equipment (cleaned in the area)
- small equipment that leaves the area (cleaned in the washing facility)
- disposable equipment (disposed of during line clearance).
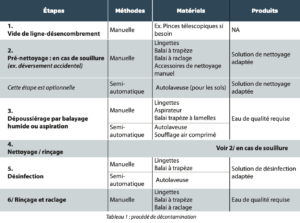
In a global approach, whatever the type of area (classified and unclassified), a decontamination process can be separated into 6 steps (for the open surfaces of spaces or the exteriors of production equipment for example). (See Tab 1)
1. The Steps
1. Decluttering
to remove all material, equipment or waste that would reduce access to the areas to be cleaned.
2. Precleaning
to remove and contain accidental soiling (during spillages), using cleaning agents specific to the type of soiling to improve efficiency. This step is generally performed during production in line with HSE and environmental constraints (e.g., a spillage in a grade B area could be handled at the end of the shift). This precleaning may be followed by rinsing and scraping of the area in question.
3. Dust removal (optional depending on the state of cleanliness of surfaces)
which allows the removal of accumulated dirt during production steps so enabling the removal of most of the soiling present.
4. Cleaning
With the aim of removing residues not captured by the previous steps. Combined with a mechanical action, there are two coexisting practices to solubilize residues:
- In the first instance, cleaning with water where water is enough to remove the residues, followed by scraping to remove them,
- Cleaning with a cleaning agent followed by rinsing (to remove the cleaning agent) then scraping.
Even if cleaning is an essential prerequisite to the disinfection step, rinsing the cleaning agent may nevertheless be performed periodically: the frequency is to be justified by changes in the appearance of the surface (e.g.: presence of traces after several cleanings, sticky or slippery surface condition,…). A visually clean and dry condition is expected at the end of cleaning before the disinfection step.
5. Disinfection
Disinfection aims to reduce the microbial load of the surface via a chemical action with a disinfectant. This disinfection step should provide a broad-spectrum antimicrobial action and should be coupled with a bio-decontamination action: sporicidal disinfection (ASD may be used as a sporicidal action). Alternating disinfectants (one of which is the sporicidal agent) is then necessary; the periodicity of the alternation is to be defined in relation to microbial pressure and the production activity of the area. This alternation of the disinfectant mode of action, via the use of different modes of action, will avoid the selection of some microorganisms.
In the particular case of the start-up of a new area or the collection phase of environmental monitoring data (that is the qualification phase), the reasoning may be as follows: alternation with the sporicidal agent is introduced arbitrarily. Depending on the results of environmental monitoring, this alternation frequency may be reviewed and adapted (e.g.: biweekly or bimonthly).
The Rules Governing Medicinal Products in the European Union Volume 4 EU Guidelines for Good Manufacturing Practice for Medicinal Products forHuman and Veterinary Use.
6. Rinsing and scraping
whose frequency is also to be defined (on the same principle as the rinsing associated with cleaning agents) aims to remove disinfectants. Systematic rinsing is expected between the use of two disinfectants. The rinsing step depends on the disinfectant used, some biocidal agents such as alcohols or hydrogen peroxide, do not require rinsing/scraping.
It should be noted that alcohols (isopropyl alcohol or denatured alcohol) can even be used to rinse other disinfectants (in place of water of the required quality).
2. Methods
To begin with, the method is applicable for all surfaces described previously (e.g.: small and large surfaces, the exteriors of equipment, etc.). The implementation of a decontamination method requires the involvement of the staff responsible for these operations, to facilitate their compliance.
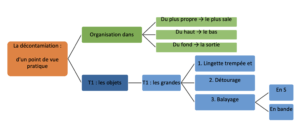
Decontamination principle for a space or equipment item:
- From the cleanest to the dirtiest
- From the top to the bottom
- From the bottom of the space to be treated (part or isolator) to the exit
Decontamination for fixed objects which are on walls (ventilation grid, spotlights, small equipment):
- Execute the detergency/disinfection procedure for small equipment with “détourage” (cleaning around the edges of a fixed object),
- Then, execute decontamination of the large surface.
Decontamination method for a room or equipment item:
- Wet wipe wrung out manually. Acceptance criterion: no more dripping of cleaning solution during wringing out;
- “Détourage” (cleaning around the edges of items);
- S shape sweeping (“sculling” method) or strip sweeping (with overlap). From an ergonomic point of view, either method may be envisaged if it encourages compliance on the part of the cleaning staff.
Sequence for small surfaces
Small non-mobile fixed surfaces such as the exteriors of tanks, fixed table, isolator hood (etc.) are to be incorporated into the cleaning procedures either for large surfaces or for small surfaces. These procedures also include small mobile equipment that remains in the area (scales, vacuum cloches, IPC equipment, etc.).
This choice is to be made using common sense and the logic of the procedures of the area. As a minimum, a sequence is to be defined for these small, fixed surfaces.
Small surfaces may be rinsed with alcohol unlike large surfaces which pose problems in terms of HSE (operator exposure); pay attention however to the questions of the compatibility of materials and surfaces.
Here the principle is the same as for spaces:
- The detergency/rinsing sequence and disinfection must be integrated.
- A sporicidal action (bio-decontamination) must be incorporated into the cleaning/disinfection sequences of these materials.
Cleaning of the surfaces of fixed equipment/furniture is carried out mostly by hand. Interventions in grade A environments must be limited and maximally controlled, with the aim of keeping an ultra-clean environment and so limiting the cleaning effort in order not to pollute the area.
3. Equipment
This paragraph aims to detail, in a general and non-exhaustive manner, the equipment that can be used for the decontamination of production environments.
a) Scrubber-dryer
The use of scrubber-dryers is to be considered provided that a specific operating method has been defined for cleaning, storage and maintenance (change of water / detergent containers, brushes, etc.); for unclassified areas and for areas classified C-D. For grade C areas, it is recommended that the compatibility of the scrubber-dryer with the environment be verified. The compatibility criteria may be as follows: equipment that is easy to clean, with low discharge (a discharge study is recommended for the step), etc.
b) Brooms
Broom (trapeze type)
+ single use or reusable wipe (solely for unclassified areas and areas classified C-D).
- Reusable cleaning equipment (e.g., broom): a detergency/disinfection procedure must be incorporated in the same way as for the small equipment of workshops (e.g., in the case of a breach of sterility and before resuming production, the cleaning equipment is subject to the same procedure as the workshop’s small equipment).
- Reusable wipe: a detergency/disinfection procedure is to be defined according to the risks identified in the area where it is used. Likewise, a traceability procedure is to be put in place to define the number of washes carried out per wipe. So, the maximum number of washes that the wipe can undergo with regard to the acceptable particle release rate will have to be defined.
Comment: the analysis of flow from the wipe is to be studied according to the criticality of the areas where it is used.
In the case of rinsing
Broom (with flaps) + wipes impregnated with water of the required quality:
Regarding water quality:
- Drinking water may be used for removing dust from unclassified areas.
- Purified water may be used for areas classified C and D (may be water of lesser quality, if regular monitoring confirms this).
- WFI water or sterile water must be used for areas classified A and B.
With regard to wipe quality
- 100% polyester wipe
– Designed specifically for absorption (knitting, mesh, etc.)
-Widely recommended in grades A/B.
- Cellulose-polyester/non-woven wipe
-Good absorptive power (quantity and speed)
-Correct return of liquid
-Correct particulate cleanliness (attention to the fibers)- Correct resistance to abrasion
- Knitted 100% polyester wipe
-Good absorption but slower than cellulose-polyester
-Good return of liquid
-Good to very good particulate cleanliness (limited risk of contamination: fibers)
-Good resistance to abrasion
In in a fairly schematic manner:
- Cellulose-polyester wipes are very efficient for particulate decontamination steps (wet sweeping).
- Polyester wipes are recommended for disinfection, with return of disinfectant liquid and efficient in cleaning.
- In grades A/B polyester wipes are preferred for reasons of particulate cleanliness and resistance to abrasion.
- In certain particular cases, we may opt for a sterile cellulose-polyester wipe (e.g., for drying).
- In grade C/D, a cellulose-polyester wipe may be proposed for cleaning and a polyester wipe for disinfection
Broom for scraping
This broom is used for the scraping detailed in paragraph 1.6 “rinsing scraping”.
4. Products
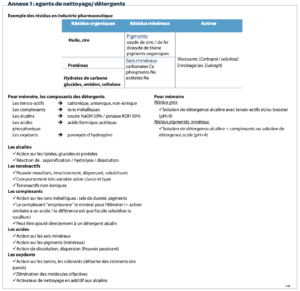
The choice of cleaning product is to be justified by the manufacturer. A list of detergent and biocidal products used in each of the areas may be created.
Cleaning with water
In the first instance, it is recommended that the possibility of carrying out the cleaning operation with water only be evaluated (at a given temperature).
How do we determine that cleaning with water is sufficient?
According to the level of surface soiling, according to the solubility of the residues to be cleaned, the use of water only may be envisaged. For this, tests may be conducted on surfaces/in the production workshop (for example). The aim is to achieve a dry and visually clean surface condition; depending on the grade of the area microbial decontamination should be carried out in a second phase in the case of cleaning with water, the rinsing operation is not required (rinsing having the aim of removing a detergent when it is used).
Comment: Cleaning with water also includes cleaning with steam.
Cleaning with a detergent
The type of detergent is to be adapted to the type of soiling met with in the pharmaceutical industry. Schematically:
- Inorganic residues: use of acidic detergents.
- Inorganic residues: use of alkaline detergents.
Note: that an alkaline detergent containing complexing agents and surfactants cleans the organic and inorganic fractions of the soiling. All these ideas are detailed in Annex 1: composition of detergents, application according to detergent type. In the context of cleaning the production environment, the idea of contact time is not applicable; the detergency procedure is to be defined as being until a visually clean surface is obtained. The frequency of the detergency phase may be subject to certain application criteria: type of activity in the workshop (type of soiling), traffic in the area (case of an airlock), etc.
Cleaning with a detergent/disinfectant
The appropriateness of the use of a combined detergent/disinfectant will depend on the spaces and the associated hygiene plan. A detergent/disinfectant product may be used either for its detergent action, or for its disinfectant action. This action is followed by rinsing/scraping (systematic or periodic).
Disinfection. The choice of disinfectant and its mode of application is to be defined according to:
- The surface type: Small/large surface (for example in light of acceptability/HSE risk),
- The compatibility between the biocidal agent / materials encountered in the area (e.g., chlorine is a corrosive biocidal agent),
- On the area in question (grade A-B/C-D/unclassified area): sterile/non-sterile biocidal agent; ready-to-use or to be diluted; wipe or spray,
- According to the endogenous strains, detected in the area and therefore the type of action to be sought (fungicidal, bactericidal ,…),
- …
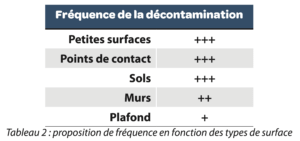
5. Frequency
Disinfection frequency must be rationalized and defined according to activities, the results of environmental monitoring (via risk analysis for example). Likewise, its periodicity may be associated with the workshop’s activity level for example or the contamination level (microbial and particulate) generated during production.
By way of example:
- Ceiling and top of the walls: every 6 months
- Wall: every 3 months
- Floor: every week
- Small surfaces (e.g., bench): every day/at the end of each campaign
- Contact points (handles, switches, etc.) may be associated with a previously described category
Table 2 Proposal for frequency according to surface type
Decontamination steps do not all follow the same frequency, that is, it is not imperative to have systematic disinfection/bio-decontamination after every cleaning. The frequency of each of these actions must be rationalized according to control of the environment and activities.
For example:
6. Conclusion
This article details the methods and materials used during the decontamination process emphasizing the importance of cleaning (visually clean), before passing to the disinfection step. It remains the responsibility of the manufacturer to document through validation of the environment that this process achieves the targeted efficiency (microbiological /particulate level according to grade and the type of activity).
Share the article
References
• GUIDELINES The Rules Governing Medicinal Products in the European Union – Volume 4 EU Guidelines for Good Manufacturing Practice for Medicinal Products for Human and Veterinary Use
• Annex 1 Manufacture of Sterile Medicinal products Aug 2022
Glossary & abbreviations
- ASD Airborne Surface Disinfection
- WFI Water for Injection
- CIG Common Interest Group
- HSE Health Safety and Environment
- IPC In Process Control
- NA Not Applicable
- SME Subject Matter Expert





Audrey THIERY
See profile on
![]()