October 2023
La Vague n°79
Special A3P Congress 2023 : Annex 1, Environmental and process monitoring
Table of Contents
- EU GMP Annex 1 (2022): Aseptic Process Simulation (APS). New challenges for the pharmaceutical industry?
- How to evaluate pyrogenic risk in an injectable pharmaceutical process? Decision-making tool
- Introduction to the dual chamber systems (DCS) filling process
- Implementation of decontamination processes in the pharmaceutical industryentation of decontamination processes in the pharmaceutical industry
- Container Closure Requirements in the New EU GMP Annex 1 - Enabling Compliance with a Holistic Science-Based Approach
- Improving the efficiency & reliability of AVI qualification: Determining the optimal number of runs with the KNAPP method
- Case Study: Effective Sterile Powder Transfer for Parenteral Drug Products
Introduction to the dual chamber systems (DCS) filling process
In the field of injectable products, particularly biologics, the proportion of lyophilized products has become increasingly large in recent years. In fact, biological medicines, such as monoclonal antibodies and vaccines are increasingly present in today’s pharmaceutical industry. Lyophilization is often used to stabilize temperature-sensitive products, which contributes to the growth in lyophilization.
Although vials are commonly used, the dual chamber system is becoming progressively more popular and provides more reliable administration conditions, which creates a real and decisive advantage for the patient. The dual chamber system is liable in the future to become the first choice in the field of self-administered injectables.
1. Basic concept
For some injectable medicines, in particular protein products, water increases the physical-chemical instability of the product. In order to prevent proteolysis or other adverse reactions, it is necessary to remove as much water as possible from these medicines.
Currently, lyophilization technology is the most widely used drying method in the world. The aqueous medicine is frozen in its primary packaging below the freezing point, then the system is placed under vacuum to directly sublimate the ice from the solid state to the gaseous state (without passing through the liquid state), so removing water from the reagent. Today, two different systems may serve as primary packaging: vials and dual chamber systems (DCS).
Whether vials or dual chamber systems, it is necessary to add diluent prior to injection. This process is called reconstitution. As this is a manual operation carried out by humans, this process is subject to uncertainty. It is at this level that the dual chamber system differs from the vial system.
a) Vial
A lyophilized product in a vial means that it is stored separately from the diluent. Currently, there are two ways of storing the diluent:
- in vials
- in pre-filled syringes (PFS).
When a diluent is placed in a vial, it is necessary to remove it with a syringe, then to deposit it into a vial containing the lyophilized product to be reconstituted. Once the product is mixed, it must be removed from the vial by the syringe provided for the injection. When a diluent is provided in advance in a pre-filled syringe (PFS), it is relatively simpler to use. It reduces the number of diluent removal steps, the operator has only to inject the diluent into the vial containing the lyophilized product then to remove the mixture to use it.
These two reconstitution methods present disadvantages. Firstly, when the syringe needle is inserted into the vial, particles may be cut from the stopper and fall into the syringe, and thus be able to enter the patient’s body and harm their physical health. Another point is that the medicine must generally be overfilled by 20% or more, to ensure that the exact dose is aspirated into the syringe, which leads to an increase in the cost of the medicine. Generally, multiple steps become very demanding for the user, requiring certain skills to obtain the right dose.
b) Dual chamber system (DCS)
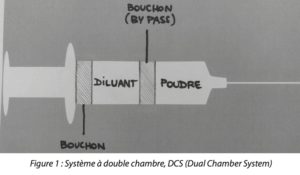
A dual chamber system is a system in which the lyophilized product and the diluent are stored in two chambers of the same container, the two chambers are separated by a rubber stopper. Today there are two types of dual chamber container on the market, the dual chamber syringe and the dual chamber cartridge. (Figure 1)
Let us take the example of the dual chamber syringe, when using it, the user has only to push on the syringe shaft and the pressure applied to the stopper between the two chambers opens a bypass along the wall between the two chambers. The diluent flows along the bypass into the lyophilized product chamber and mixing occurs. Given that all substances are pre-filled, the dual chamber system reduces excessive filling and the risk of contamination. In addition, the dual chamber system also reduces the risk of medication errors by eliminating the use of several vials. The easy reconstitution and the advantages mentioned provide great convenience for patients. As a result, the dual chamber system becomes a better choice in self-administration and emergency situations.
2. Filling process for the ready-to-use dual chamber system
The filling and lyophilization process for vials is different from that for the DCS system. Vial filling and lyophilization technology is relatively mature: washing, depyrogenation, filling, pre-stoppering, loading of vials into the freeze dryer, then lyophilization. (Figure 2)
Figure 2: Filling and Lyophilization process for ready-to-use vials
In the entire process, regardless of the type of barrier system used (oRABS, cRABS or isolator), material transfer technologies have proven themselves on the market for many years. This concerns the product or the active principle (via closed pipes, DPTE door or SART port) elastomer stoppers and caps (via DPTE door or via an airlock in double pouches). Nevertheless, things are different for the dual chamber system. Unlike a vial which presents a large contact surface for lyophilization, the dual chamber system has a smaller contact surface and its components are more complex. For example, silicone components are used so that the stopper glides smoothly. Consequently, the interaction between the silicone and the medicines must be taken into consideration in the development process. It is important to emphasize that the dual chamber syringe cannot stand on the freeze dryer shelf like a vial and must be maintained on supports. This leads to the limitation of thermal conduction during the lyophilization process and to greater reliance on convection and radiation. Consequently, the lyophilization cycle is different.
In cooperation with pharmaceutical companies, Tofflon has conducted numerous experiments based on medicines to study the impact of different shapes of syringe support on the filling and lyophilization process. These tests relate to psychotropic medicines. The aim was to study the impact of supports on the filling and lyophilization process. Because dual chamber syringes cannot stand on the freeze dryer shelves on their own, it was necessary to have a support to maintain them in position and studying the impact of different types of support on the production process seemed inevitable.
a) Experiment A
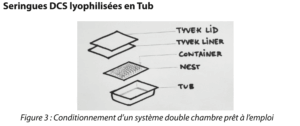
DCS syringes lyophilized in a tub
With the use of a ready-to-use dual chamber syringe, it is possible to envisage the filling and lyophilization process being conducted directly in the final packaging format, that is to push the tub filled with DCS syringes into the freeze dryer. (Figure 3)
The filling process is similar to that of the filling of pre-filled syringes, with the lyophilization process added in the middle and a second filling step after lyophilization. (Figure 4)
Figure 4: Filling and lyophilization process for DCS syringes in tubs
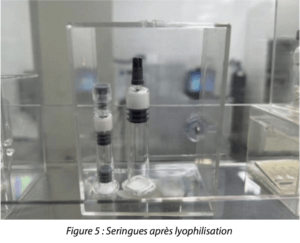
Figure 5: Syringes after lyophilization
All DCS product steps are known and verified in the factory, including the introduction of the active principle and the transfer of equipment. The disadvantage is also obvious since the tubs are generally made of polyethylene, a material with poor thermal conductivity that may require a longer lyophilization cycle.
b) Experiment B
Dual chamber syringes lyophilized on a perforated support
As the lyophilization cycle of experiment A was long, it was interesting to look for a solution to replace the tub and the nest and to be able to judge whether the lyophilization cycle could be shortened. The whole process must be carried out in a barrier system, normally in an isolator, despite the high constraints on the transfer of equipment and the operation.
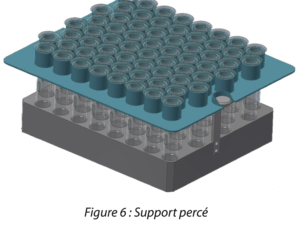
In this experiment, a stainless steel support, adapted to use under aseptic conditions, was specially designed with holes to hold the syringes. This support provides better thermal conductivity during lyophilization. (Figure 6)
Figure 6: Perforated support
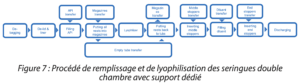
After filling, the nest containing the DCS syringes is taken out of the tub and placed in the stainless steel support, then loaded into the freeze dryer. You will be able to observe the filling and lyophilization process for DCS syringes with a dedicated support. (Figure 7)
Figure 7: Filling and lyophilization process for dual chamber syringes with a dedicated support
It was observed that at the end of testing, the lyophilized powder did not collapse and the moisture content was within the qualified range. There was also no powder in the dilution chamber. These observations led to the conclusion that this process produced a satisfactory result, compared to the process with the nest. (Figure 8)
Figure 8: Result of the lyophilization process for dual chamber syringes in a perforated support
In comparison with the process used in experiment A, two additional steps must be considered: the transfer of the stainless steel supports and the transfer of empty tubs. These steps must be carried out in a sterile environment, which increases the surface area of the barrier system. In addition, because of the open-hole structure of the stainless steel supports, it is necessary to provide a suitable process for cleaning and sterilizing the support.
c) Experiment C
Dual chamber syringes lyophilized in sleeves
Figure 9: Sleeve support
In order to reduce the loss of energy in the thermal conduction process, another support was designed. It is presented in the form of a sleeve with a thinner wall. This type of sleeve is not in the traditional cylindrical shape, its lower diameter is larger to increase the contact surface area between the bottom of the sleeve and the freeze dryer shelf. This prevents the syringes from tipping over when they are loaded. The height of the sleeve is enough to guarantee that the syringes are held securely. (Figure 9)
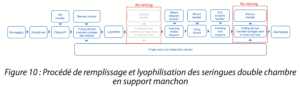
Just as with the procedure for the perforated support, it is necessary to remove the tubs and syringes from the nest after filling, then to transfer the syringes into the sleeves. (Figure 10)
Figure 10: Filling and lyophilization process for dual chamber syringes in a sleeve support
The DCS syringe is completely closed by stoppers at the end of this process. The syringes may be re-nested after lyophilization or at the end of the process.
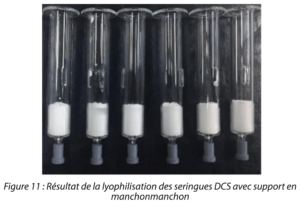
At the end of the lyophilization test, the results proved positive: no apparent collapse of the product, satisfactory moisture content and no trace of powder in the dilution chamber. (Figure 11)
In comparison with experiment B, the entire process of experiment C presents enormous advantages. Firstly, the lyophilization cycle is much shorter. Then, it is possible to place the re-nesting step at the end of the process (after complete closure of the syringe). This may shorten environmental exposure time and reduce grade A space requirements, as re-nesting can be carried out outside grade A. Finally, the sleeve can be cleaned and sterilized using the vial washer and the depyrogenation tunnel. The washer and the tunnel can be connected directly to the filling line to obtain complete line automation.
Figure 11: Results of the lyophilization of DCS syringes with sleeve support
Due to the materials and the shapes of the different supports, their effects on thermal conductivity are also different. The tubs and the perforated supports are large and rectangular, they can be arranged regularly on the freeze dryer shelves. Conversely, the sleeves are cylindrical and can be arranged in staggered rows on the shelves. In other words, it is possible to place more syringes on the same shelf using sleeves as supports.
Through the testing of these three different types of support and according to the effect of the lyophilization cycle and of the occupation level of the freeze dryer shelves, the sleeve support solution is more advantageous than the other two supports tested particularly for products that are difficult to lyophilize. However, judging by the complexity of the filling process and the challenge of the barrier system, direct lyophilization in a tub is preferable to the perforated support and to the sleeve support for products that are easier to lyophilize.
d) Experiment D
Direct filling of lyophilized powder
Figure 12: Direct filling process for lyophilized powders
Although the lyophilization processes mentioned above are all feasible, they all entail constraints, as the nature of the product itself impacts the effect of the lyophilization. In other words, some products are easy and others are difficult to lyophilize.
A large number of lyophilization tests showed that some product and support combinations are sensitive, generating a smaller “design space”. Consequently, the water content sometimes exceeded the norm.
Alternative approaches may however be implemented to better manage moisture content in the filling and lyophilization process.
In the first instance, the bulk lyophilization process must be used, then an isolator process to control the temperature and humidity of the environment. After lyophilization, the powder must be filled and the containers sealed as soon as possible, to minimize the exposure time of the powder. Regarding filling, there are two ways of proceeding: the filling of bulk powder and the filling of powder in the form of spheres or blocks. Bulk powder has “free” particles while powder blocks and spheres are more solid, rather like chalk. The different forms require different types of preparation and different dosing methods. In all cases, if the powder is deposited directly into the syringe, the overall process is similar to the filling of powder in vials, and there are ready-to-use technologies for the aseptic transfer of powder, stoppers and diluent. (Figure 12)
For the filling of powder blocks or spheres, the other process steps are similar to those of bulk powder filling, but the filling method must be carefully designed. During filling, the integrity of the powder blocks and spheres must be maintained, otherwise the dose may be insufficient, or product may be wasted, with economic loss.
Direct filling of powder allows for a lyophilization process that is much simpler than the methods mentioned above.
In fact, direct filling of powder avoids the DCS syringe entering the freeze dryer, and also avoids the calorific loss caused by the syringe support (during the product cooling phase, the heating phase and the sublimation phase). It also avoids the step where the syringe is introduced into the support. Nevertheless, this poses a greater challenge for the filling process.
Figure 13: Dual chamber vial
3. Extensions & market
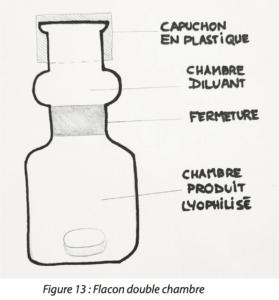
Dual chamber systems comprise of vials, syringes and cartridges. Although it is still rare, the DCS system still has its place in the market. For example, Pfizer has chosen to package some anti-inflammatory agents in DCS vials. (Figure 13)
Unlike syringes and cartridges which have a cylindrical shape, DCS vials have separate upper and lower chambers. The lower chamber keeps the lyophilized product in an atmosphere which can be inerted. A stopper in the middle separates the two chambers. The upper chamber contains the diluent. The vial is closed with an upper stopper and a plastic cap.
During use, the operator firstly presses on the plastic cap. The pressure pushes the central stopper directly into the lower chamber opening a channel so reconstituting the diluent with the lyophilized product. Then, the operator removes a little tab covering the center of the upper stopper, inserts a syringe and removes the mixture.
Compared with traditional vials, dual chamber vials offer reconstitution conditions in situ. But in comparison with the dual chamber syringe, this always requires an additional syringe to remove the mixture and does not avoid the problem of the needle cutting the rubber stopper.
Currently, the dual chamber system is mainly used to package medicines for self-administered treatments such as hemophilia, schizophrenia, diabetes, erectile dysfunction, endometriosis and precocious puberty. The size of the global market for dual chamber syringes was 131 million USD in 2020, rising to 191.3 million USD in 2022. The size of the dual chamber system market is increasing regularly driven by the growth in biotech and lyophilized products. The size of the market should reach 4.14 million USD in 2029 according to projections.
4. Conclusion & future
We carried out thorough tests with a variety of medicinal products, for each lyophilization method for dual chamber syringes. Through these tests we saw that there is no one solution for all uses. Different medicines require different methods.
Any method may encounter failures of the lyophilization process or production steps that cannot be automated. We should take account of many aspects such as the characteristics of the medicine, the surface of the barrier system, the cleaning and sterilization of materials and the compliance of production process operations. These elements cannot be handled by a single pharmaceutical company or a single pharmaceutical equipment manufacturer but require joint research by different stakeholders to improve the process.
From Tofflon’s point of view, the risk in using traditional vials is concentrated rather on the medicine administration step, which is equivalent to leaving the risk to medical staff or patients. The risk of the dual chamber system is the reverse, concentrated on the development and medicine production stages, leaving the risk to the equipment manufacturer and to the pharmaceutical companies.
In the area of self-administration and emergency medicine, practical use scenarios make the dual chamber system more appropriate than vials. In the near future, as the dual chamber system production process becomes more mature, it will possibly replace vials.
Share the article
References
- [1] Joerg Zimmermann. Comparing Lyophilization in Vials and Dual-Chamber Systems.
- [2] Vetter. Vetter dual-chamber technology.
- [3] Joerg Zimmermann. The lyophilization process in a vial versus dual-chamber syringe: What’s different? What’s the same?
- [4] Pfizer Hospital US. https://www.pfizerhospitalus.com/products/solu-cortef
- [5] Double-chamber vials.
- [6] Marketsandmarkets. Dual Chamber Pre-filled Syringes Market. Https://www. marketsandmarkets.com/Market-Reports/dual-chamber-prefilled-syringes- market-132336702.html
- [7] MarketWatch. 2023 Double Chamber Prefilled Syringes Market Size and Gross Margin with [91 Pages] till 2030 | Report by Absolute Reports. https://www.marketwatch.com/ press-release/2023-double-chamber-prefilled-syringes-market-size-and-gross-margin- with-91-pages-till-2030-report-by-absolute-reports-2023-04-27
- [8] The Zigverve Team. Examining the Mind: What Are the Top 3 Risk Factors That Can Lead to Drug Abuse?
Glossary & abbreviations
- DCS Dual Chamber System
- PFS Pre-FilledSyringe
- oRABS open Restricted Access Barrier System
- cRABS closed Restricted Access Barrier System USD United States Dollar

Edison ZHU
.