1. Quality Systems: ensuring product compliance
Conscious of their obligations with regard to the public, pharmaceutical companies hold themselves to ever stricter ethical standards in order to guarantee the safety and quality of drugs throughout their lifetime, from research to commercialization. Patient safety and compliance with regulations are major challenges.
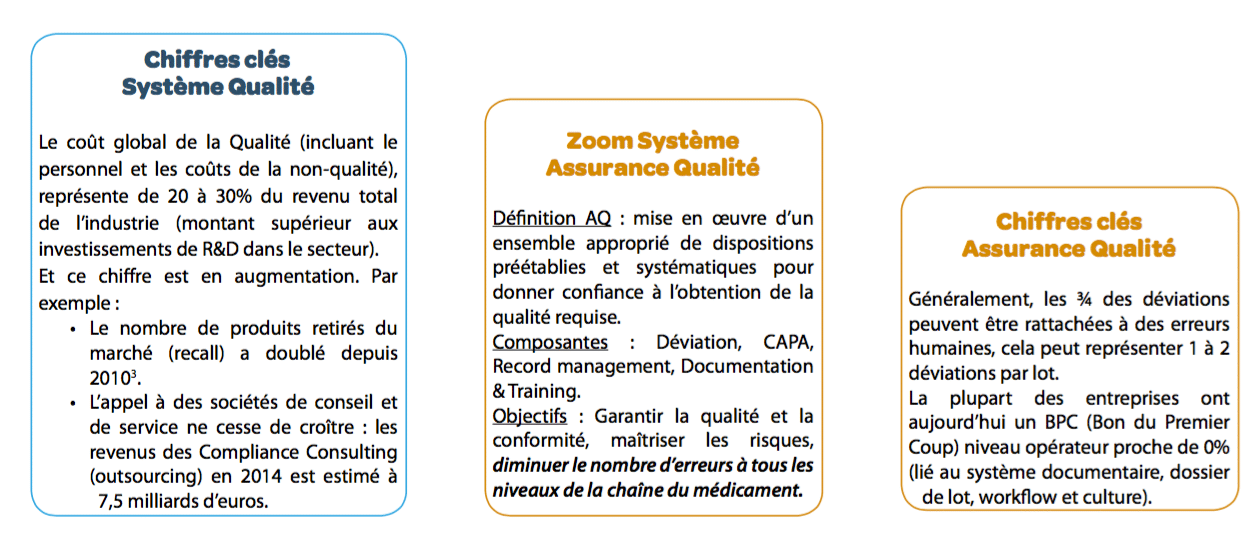
Over the last 25 years, Quality Systems have been constantly changing: frequent modifications to regulations, growing internationalization of medications, etc. Pharmaceutical quality systems must be increasingly effective to ensure risk management at all stages of the drug value chain. These systems become more and more complex and are thus ever more costly, as well as increasingly ineffective (increased sorting, deviations,drug shortages, etc.) It is important to rethink quality systems: process mastery, skills development, team involvement in quality, document system optimization, etc.
Complex, costly, ineffective…. Why do companies struggle so much to get it ‘right first time’?
But how can documentation and training contribute to managing the operator’s risk and reducing errors at the shop-floor level?
2. Documentation: First lever for mastering daily operator activities
As detailed in the Good Manufacturing Practices, “Good documents are an essential part of the quality assurance system. Clear texts prevent errors inherent in verbal communications. […] The legibility of the documents is of paramount importance.” Because of audit comments, incorporation of corporate demands and failure to manage document creation, documentary systems sprawl.
But what is the purpose of the documentary system? How are all these documents used?
Documentation today: low added value?
Traditionally, documents were considered as tools to meet regulatory requirements: documents were “intended for inspectors.” This vision had consequences in the field, including:
- content ill-suited to the reality at the shop-floor level (dense, visually poor documents),
- documents consulted rarely/not at all by production employees,
- “off-system” documents created in parallel to the documentary system,
- a verbal culture to train teams, based on people and not on systems.
In conclusion,
- Documentation is considered a necessary evil by operational staff: it does not allow for autonomy; meanwhile, training and standardization of practices is difficult. A verbal culture predominates, often leading to non-uniform practices, deviations, human error and rejected batches!
- The documentary system becomes increasingly costly to maintain: declining efficiency despite major efforts, continuous improvement difficult or even impossible to achieve. The cost for one document could be of 3.500€.
- Errors persist and performance declines in the field (rejections, deviations, human error, etc.).
A new vision: considering documentation as a working tool
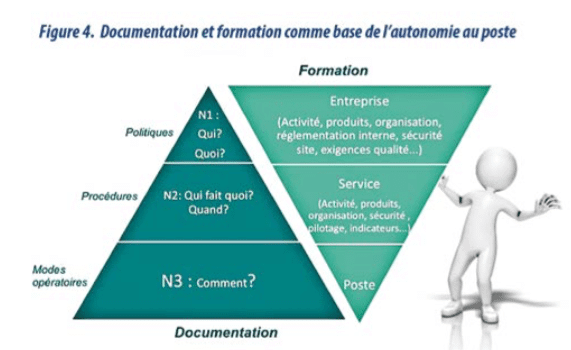
With an innovative vision, documentation is seen as the operator’s third working tool (after machines and raw materials). (diagram 1) It is time to design our documentation in a more efficient way, to increase its value, and avoid human error and QA costs. SOPs are not only documents, but information to be provided to doers, to help them do their daily work.
Placing the operator at the center of the quality system
Users become central: they must have quick and easy access to the critical information they need. To this end:
- The information is structured based on the process map, identification of the critical stages and the document pyramid.
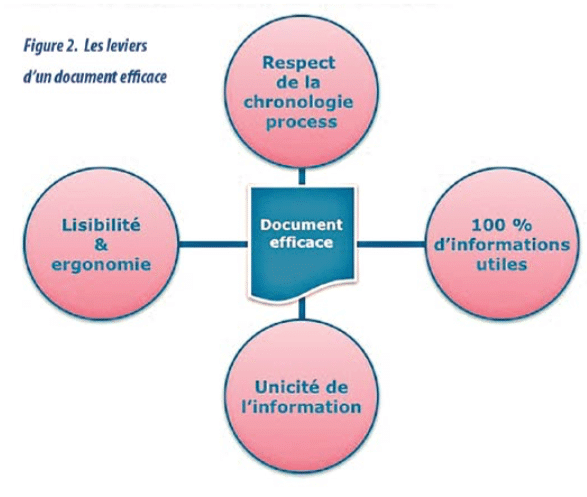
- Documents comply with the basic rules of ergonomics, legibility, etc (diagram 2). and innovative media are employed (“IKEA”-like instructions, touchscreen tablet, video, etc.).(diagram 3)
the objective is of course to enhance documentary efficiency The O.D.E. (Overall Document Efficiency)5 indicator was developed with this in mind (you can only manage what you can measure!). Although the O.D.E. is currently close to 12% in companies (i.e. operators have approximately one chance in 10 of finding the information they need at the time they need it), this new approach makes it possible to achieve an ODE greater than 70%.
Taking control of document creation
In parallel, creation of documents must be placed under control:
- by working on the link with CAPAs, deviations and Change Control;
- by avoiding inopportune document modifications and creations, and instead analyzing the criticality and relevance of requests;
- by defining and implementing the necessary skills within the company (e.g. by training document writers).
3. Training: a logical extension of the documentary system
The documentary and training processes are closely linked: formalizing the company’s know-how to facilitate transmission.
What is effective training? How can training efforts be targeted?
Training today: long and costly
Training initiatives observed in companies today are not very effective.
All companies aim to increase the quality culture and performance of their employees. Some deploy training/education programs in the hope of enhancing employees’ knowledge. However, the results are often the same:
- the content approach is inappropriate;
- The pedagogical approach is not proper with training too theoretical
- training is not performed at the right time;
And from a performance point-of-view:
- many hours are spent in training,
- staff do not gain autonomy quickly
- and the objectives of the training are rarely achieved
Training in the future: targeted, offering rapid autonomy
Placing the operator at the center of the system
As with documentation, the user becomes central. The content, approach, timing, etc. must be designed with the end user in mind. To do this, the right questions must be asked:
- Content: Who needs to be trained? For what scope? What is the relevant content according to trainees’ profiles and skills?
- Approach: What is the best pedagogical approach depending on the training objective? Does this approach suit trainees? At the end of the training process, do trainees feel confident to perform their work alone?
- Timing: Is it performed at the right time, not too late, not too early? Do trainees implement the tasks for which they have been trained within a short timeframe?
The objective is to focus on the ability to perform the work alone after training As with documentation, work must be done to redesign training with the aim of increasing training efficiency for the operator. Today, the O.T.E. (Overall Training Efficiency)6 indicator makes it possible to measure training efficiency. The average O.T.E. currently observed is around 10% (i.e. an operator has approximately 1 chance in 10 of carrying out the task at the end of the training process correctly and without error). This new approach would increase training efficiency to 60%
Structuring the training process is an indispensable step for managing training within the company:

- Identify a “process owner” for the training;
- Optimize (or define) the training management process itself: identify needs, determine the training strategies, design the training modules, implement training and track progress, etc.;
- Set out the guiding principles: frequency of re-training? Training of trainers? What impact does prior experience have on training, etc.….?
4. Major challenges in a context of cost reductions…
A company’s documentary structure may be seen as the reflection of its organization “control window” based on practice written and oral
this information structure must be perfectly connected with the perimeters of knowledge to be transmitted. Users see the benefits of having user-friendly documents suited for immediate use to make it possible to transmit know-how to new arrivals within a company. The purpose of both documentation and training is, after all, to ensure the quality, effectiveness and reproducibility of everyday work:
The stakes are therefore very important, with several savings opportunities:
- fewer “human errors” and thus deviations, batch rejections or stock shortages, etc.;
- a reduction in documentary volume and the cost of documentation and training maintenance;
- a process appreciated by regulatory compliance inspectors (some pharmaceutical companies present the changes in their ODE during inspections).
5. Summary documentation and training as pillars of operations autonomy
In conclusion, thought must be given to everything users need to get it right first time:
- training that must enable users to acquire the knowledge and know-how they need;
- easy and immediate access to necessary information. Documentation and training are pillars of on-the-job autonomy .
This approach must be extended to all systems surrounding users such as non-compliance management, simplification of batch records, process control, etc. Furthermore, guidance must be provided to ensure that these changes are fully understood and accepted. (diagram 5)
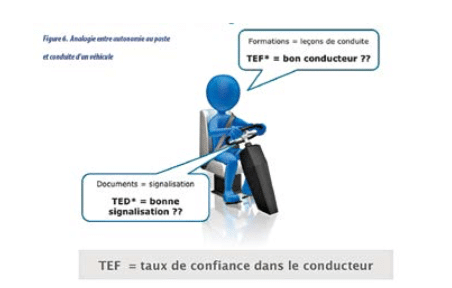
Documents can be seen as a car airbag: it is used only rarely, but in the event of a problem it must work immediately! Training can be compared to driving lessons.
Do you have confidence in your documents and training? Do you have confidence in your airbag and driving lessons? Would you get into the vehicle?

Camille MARCEL – ALTRAN
camille.marcel@oxo-group.com
Share article
References and glossary
O.D.E., Overall Document Efficiency rate (ODE) is an innovative measure developed by Altran World Class Center Lifesciences Process Excellence to assess the percentage of chance that the operator has of finding the right critical information in a document, at when he needs it.
O.T.E., Overall Training Efficiency rate (O.T.E., Overall Training Efficiency rate), is an innovative measure developed by Altran World Class Center Lifesciences Process Excellence to quantify the share of people who perform their activities correctly at the end of the training process.
Source FDA, McKinsey